Abstract
Background: The growing importance of patient preferences in treatment decision-making in oncology is evidenced by the expanding role of patient-reported experience in both regulatory and reimbursement considerations of value. While recent introduction of new treatments for multiple myeloma (MM) have demonstrated longer time to progression and improved survival, specific regimen options still vary with respect to efficacy, safety, and dosing. Therefore, patients and providers must consider the trade-offs inherent in making treatment decisions. However, a lack of evidence exists describing patient reported preferences within the context of currently available regimens. To address this gap, this interim analysis of an ongoing study was conducted to examine patient preferences for MM treatments.
Methods: A sequential mixed methods design, which incorporates both qualitative and quantitative phases, was utilized for this study. The qualitative phase identified content and language, via semi-structured interviews, to elucidate how patients understand and construct treatment-related factors, such as overall survival (OS), progression free survival (PFS), dosing, and tolerability. Results from the qualitative phase were subsequently used to inform the development of the quantitative survey. The online survey was sent to adults diagnosed with MM, who had received or were currently receiving first-line therapy (FL), second-line therapy (1PL), or third-line therapy (2PL) at the time of the survey (target N=200). Patients were recruited from targeted panels, advocacy partnerships, patient communities, and physician referrals from May to June 2018. The survey utilized a Discrete Choice Experiment (DCE) methodology to assess preferences and willingness to accept trade-offs among hypothetical treatments that varied on levels of specific attributes. Treatment attributes and levels were identified through literature review, current treatment guidelines, and clinical input. In the quantitative survey, patients were asked to rate the levels of each treatment attribute (based on a 5-point Likert scale, ranging from 1=very bad to 5=very good) and select which regimen they prefer when presented with two different hypothetical regimens (fixed choice exercise - Table 1) to identify trade-offs that patients were willing to make when selecting treatments. Descriptive statistics were used to characterize the interim survey data. Full DCE results, which will model additional treatment scenarios, will be based upon the complete sample.
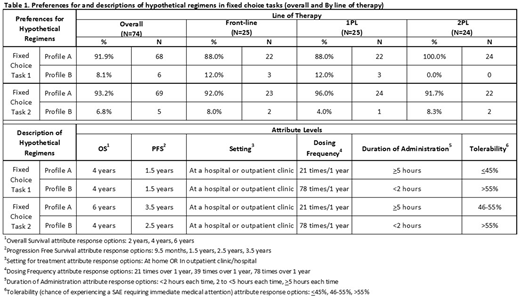
Results: In this interim analysis (n=74), the mean age was 63 years and 51% were male. The average time since diagnosis was 70 months. Twenty-five patients were on FL, 25 were on 1PL and 24 were on 2PL. Mean duration of most recent/current treatment at the time of survey completion was 16 months for FL, 15 months for 1PL, and 11 months for 2PL. On average, patients in earlier lines of therapy (FL and 1PL) indicated greater importance of OS than 2PL patients. The importance of tolerability was also greater for patients in earlier lines of therapy (FL and 1PL vs. 2PL). Results of the fixed choice exercise available at the time of this analysis are shown in Table 2. When efficacy (OS and PFS) were comparable, 92% of patients preferred the treatment profile with a lower dosing frequency over a 1 year period (21x) despite a longer infusion time (>5h) compared to a treatment profile with higher dosing frequency (78x) and shorter infusion time (<2h).
Conclusion: Results from this interim analysis suggest that patient preferences for MM treatments may vary by treatment history. Additionally, when efficacy is similar, a significant number of patients place greater importance on dosing frequency than on the duration of treatment administration. Patients may consider treatment options holistically - e.g., convenience is not simply "chair-time," but rather also includes frequency of outpatient visits. This study provides insight into how patients with MM value and assess meaningful "benefit-risk" when making treatment decisions, which can be useful for facilitating physician-patient communications and shared decision making. Analysis of the complete study population and DCE will provide additional results on the relative importance of specific treatment attributes and preference for hypothetical treatment regimens that were not available for this analysis.
McKay:Janssen Scientific Affairs,LLC: Employment. Maiese:Janssen Scientific Affairs,LLC: Employment, Equity Ownership. Chiarappa:Janssen: Employment. Cambron-Mellott:Kantar Health: Employment. Maculaitis:Kantar Health: Employment. Alunni:Kantar Health: Employment. Raje:BMS: Consultancy; Celgene: Consultancy; Janssen: Consultancy; Merck: Consultancy; Takeda: Consultancy; AstraZeneca: Research Funding; Research to Practice: Honoraria; Medscape: Honoraria; Amgen Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.